Introduction to Low-Level Laser Therapy (“LLLT”) Technology
Medical researchers began using laser biostimulation in the late 1960’s with low-powered laser beams that produced non-thermal effects on human tissue. The first reported cases involved slow-healing ulcers. The efficacy of this low-level laser therapy, or “LLLT,” is substantiated by objective research that continues. Experimenting clinicians found that an 830 nm laser is optimal for treating chronic pain. An example of how LLLT works involves soft tissue trauma. These types of injuries consist of damage to the deep, sensitive layers of tissue beneath the epidermis, including muscular, neural, lymphatic, and vascular tissue.
The human body normally reacts to this soft tissue trauma by “splinting” the injury with edema, a thin or watery fluid in tissue spaces or cell interstices. However, excess edema causes swelling that inhibits movement of the damaged tissue. These injuries result in two types of pain. The first is actual traumatic pain from the injury itself, and the second pain is from the swelling that results. LLLT focuses first on the lymphatic system which maintains the body’s fluid balance, while the laser light also helps absorb the excess edema. LLLT thus provides relief in two ways.
The ML830® has FDA clearance for devices under both the “NHN” and “ILY” classifications. The ML830® was cleared by the FDA for treatment of carpal tunnel syndrome. (See “About Us”). This clearance followed a double-blind study on CTS that was conducted at General Motors.
Laser wavelengths between 820 nanometers (nm) and 840 nm have an extremely low absorption rates in human tissue. This means that laser light penetrates deeply at those frequencies.
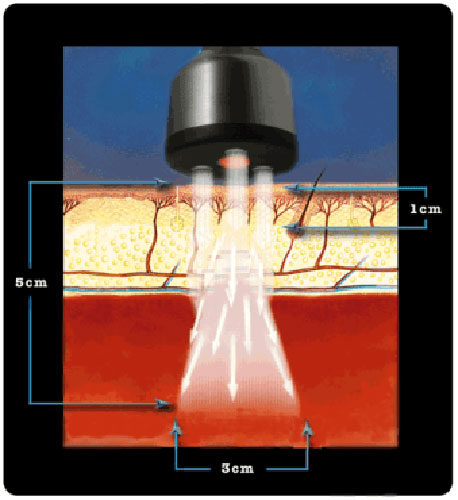
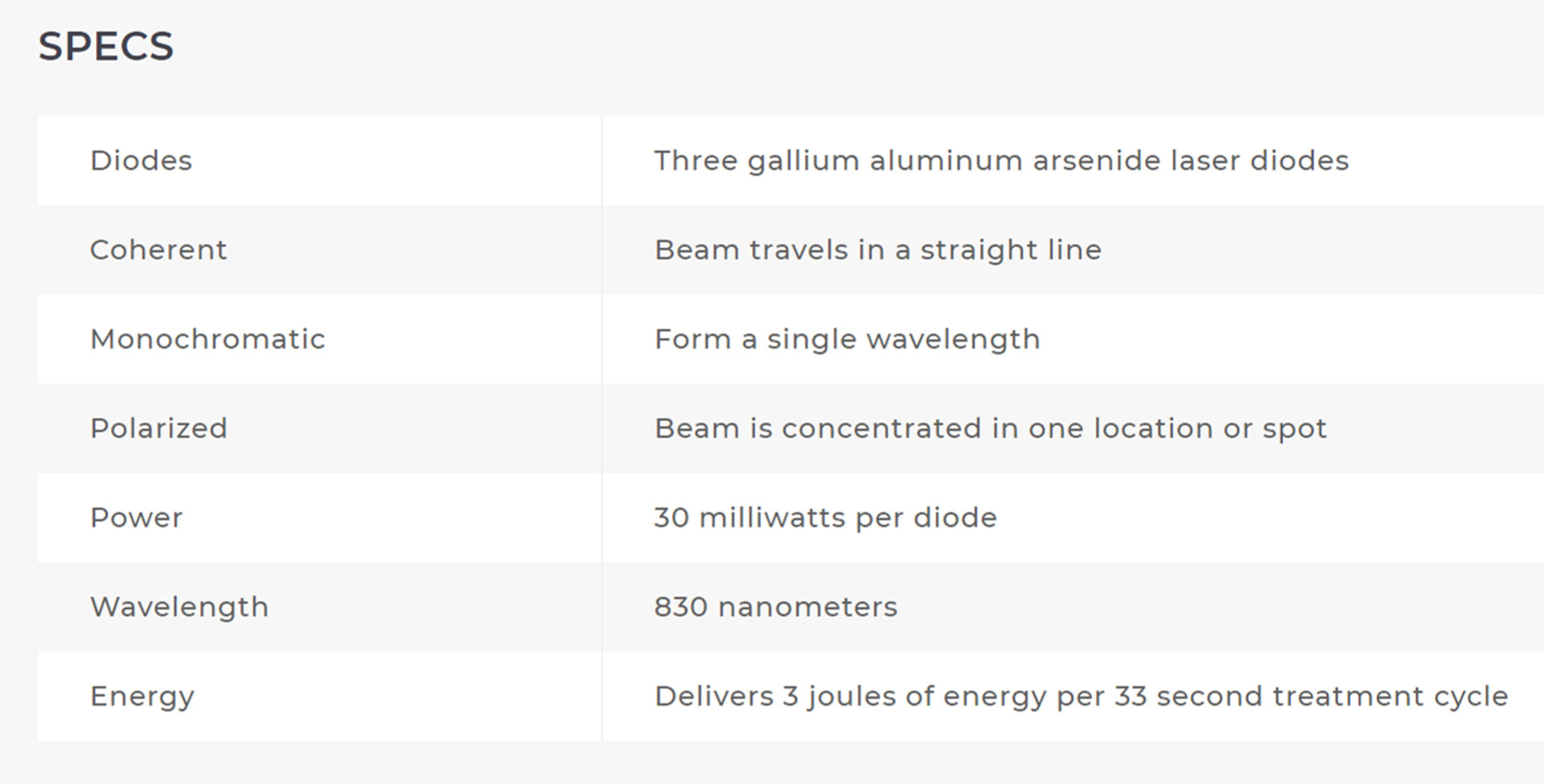
The ML830® Smart Laser is a GaAlA Laser that has wavelength of 830 nm with a power output of 90 mw. At this wavelength and power the ML830® Smart Laser has a penetration of approximately 5 cm with a 3 cm lateral spread.
It is not an accident that the the 830nm technology for its ML830® Smart Laser was chosen and patented. There are 30+ years of clinical studies that proved the 830nm range is the optimal wave-length.
General Motors Double Blind Study
General Motors had serious problems with CTS among its workers. GM responded by conducting a 36-week double-blind study, using the ML830® to see if non-invasive conjunctive therapy would help. Among the 166 afflicted GM workers who entered the program, those treated with the ML830® laser showed significant improvement in grip strength and range of motion when compared to other GM workers who were treated with placebo lasers.
A prominent medical school in Houston conducted a similar double-blind study on CTS in 1998 that showed a 70% improvement after conjunctive therapy using the ML830® among those patients in the active group.